Grrrr….once again I am running into the same old problem with way too much growth on my plates. After trying and trying again with different amounts of yogurt or milk being added into the peptone water I still cannot get countable CFUs. The first dilution used to plate was a solution of 99ml peptone water with 1ml of yogurt or milk. Then I used that solution to plate with different amounts (100µl, 75µl, 50µl, 25µl, & 10µl) on five different plates for each yogurt or milk. When the first set of plates came back with too much growth I moved onto trying again with a different amount of product. The second dilution used to plate was a solution of 99ml peptone water with ½ of 1ml of yogurt or milk. Again the solution was used in different amounts on five different plates. Still I kept getting too much growth so I went even smaller with the amount of yogurt or milk being added into the dilution. I gave it two more attempts with the amounts of 1/10 of 1ml and 100µl of yogurt or milk; believe it or not there was still too much growth.
So after realizing that this course of action was not working I went to Cori for help. She suggested that I try to dilute my dilution solution instead of changing the amount of yogurt or milk being added into the peptone water. So my new course of action is to start with 99ml peptone water with 1ml of yogurt or milk then I’ll be taking 1ml of that solution and adding it into a new peptone water then adding 1ml of that solution into another new peptone water. I’ll keep repeating this process until I reach the fifth peptone water. After I have the five dilutions I’ll be taking 100µl from each dilution and plating it. I am keeping my fingers crossed that this course of action gets me the results I need to move forward in my project.
The top picture is of the five dilution of peptone water I prepared inside the lab to see if I can get countable CFUs. The bottom picture is from http://www.personal.psu.edu/ to show a more clear understanding of what I am trying to do to get countable CFUs
S-STEM Scholar Jeanette Johnson's Blog
Saturday, November 8, 2014
Thursday, October 30, 2014
Redo week...
I don’t have too much to report this week in lab. Aside from having to re-plate because the plates were left in the incubator too long, I haven’t moved too far ahead in my project this week. I am trying to get some good/countable CFU (colony forming units) so I can compare results after putting the yogurts, buttermilk, and kefir milk through the mockup of the human stomach I’ll be making inside the lab. Speaking of the human stomach mockup I had a difficult time coming up with how to actually do something close to the stomach. After brainstorming about it and talking with Matt and Josh I finally came up with something I can do with the resource within the lab.
I’ll be using a recipe of hydrochloric acid, sodium chloride, potassium chloride, and distilled water to make up gastric acid of the stomach. After making the solution a proper amount of yogurt/milk will be added to it and then placed inside a Ziploc bag. Once inside the Ziploc bag it will then be placed flat inside a box with marbles laying on top of the bag. The whole box will then be put onto the rocking machine inside the lab so the marbles can move back and forth atop of the bag. This is what I have come up with to simulate the muscles of the stomach. I’ll be running the whole stimulation for two hours on each of the products because it usually take about two hours to digest food. I am looking forward to actually running this stimulation when the time comes. Well fellow S-STEM scholars until next time and good luck with your projects.
Picture of the redos done this week there were sixteen done in all. Four for each product with different amounts on each one. The amounts were 25µl, 50µl, 75µl, and 100µl. Using a 99ml peptone solution with 1/10 of 1ml yogurt/milk product.
I’ll be using a recipe of hydrochloric acid, sodium chloride, potassium chloride, and distilled water to make up gastric acid of the stomach. After making the solution a proper amount of yogurt/milk will be added to it and then placed inside a Ziploc bag. Once inside the Ziploc bag it will then be placed flat inside a box with marbles laying on top of the bag. The whole box will then be put onto the rocking machine inside the lab so the marbles can move back and forth atop of the bag. This is what I have come up with to simulate the muscles of the stomach. I’ll be running the whole stimulation for two hours on each of the products because it usually take about two hours to digest food. I am looking forward to actually running this stimulation when the time comes. Well fellow S-STEM scholars until next time and good luck with your projects.
Picture of the redos done this week there were sixteen done in all. Four for each product with different amounts on each one. The amounts were 25µl, 50µl, 75µl, and 100µl. Using a 99ml peptone solution with 1/10 of 1ml yogurt/milk product.
Thursday, October 23, 2014
Off to a good start...
This week in lab I started plating my two yogurts and kefir drink using the L. S. Differential media I made last week. The first set of test I did on Monday came back with too much growth on the plates. So I had to readjust the amount I was using on the plates. I first start out using 100 µl of solution on the plates but that produced way too much so I lowered the amount to 25 µl. The second set of test plates were done yesterday (Wednesday). It takes about 48 hours in the incubation process for growth to happen. I’ll be checking on the plates tomorrow (Friday).
Other than plating this week in lab I have be gathering material together to make up my human stomach simulation. After checking with Cori to make sure that the lab had all the ingredients I needed to make up my gastric acid. I got the green light that the lab has all the ingredients I needed. I’ll be using a recipe of hydrochloric acid, sodium chloride, potassium chloride, and distilled water to make up gastric acid of the stomach. I have also been working with Josh to come up with a way to react how the human stomach digest food. We came up with a pretty good way to do it but I’ll share that next blog. Got to have something to write about next week. Hope everyone’s projects are coming along nicely and I hope you all have a great weekend.
The pictures above are of the materials I used to plate this week and of the first set of test plates done on Monday October the 20th, 2014.
Other than plating this week in lab I have be gathering material together to make up my human stomach simulation. After checking with Cori to make sure that the lab had all the ingredients I needed to make up my gastric acid. I got the green light that the lab has all the ingredients I needed. I’ll be using a recipe of hydrochloric acid, sodium chloride, potassium chloride, and distilled water to make up gastric acid of the stomach. I have also been working with Josh to come up with a way to react how the human stomach digest food. We came up with a pretty good way to do it but I’ll share that next blog. Got to have something to write about next week. Hope everyone’s projects are coming along nicely and I hope you all have a great weekend.
The pictures above are of the materials I used to plate this week and of the first set of test plates done on Monday October the 20th, 2014.
Thursday, October 16, 2014
Making Media
Top picture is of L. S. Differential medium base & the bottom picture is of the antibiotic-free skim milk.
Yes after waiting and doing some more waiting I actually got to physically start my project this week in lab. All my supplies finally came in to make the L. S. Differential media I’ll be using in my project. On Monday I talked to Cori about how long it would take from start to finish. She told me that it would take about three and half hours. It was a no go on Monday for making the media because I wouldn’t have enough time to finish.
On Wednesday I came into the lab and made the media. I’m not going to lie it took me more than three and half hours to make the media. However, it was a great learning experience. I ran into a few problems making the media but nothing I couldn’t overcome with the help of Cori. The first problem that came up had to do with adjusting the timer on the autoclave machine. The L. S. Differential media requires antibiotic-free skim milk to be added into the main media along with TTC (Triphenyl-Tetrazolium Chloride). In preparation the antibiotic-free skim milk had to be autoclave for five minutes and the setting on the autoclave are set at fifteen minutes. When we asked Josh about how to go about changing the settings he brought up another way to prepare the skim milk instead of changing the settings. That way was to use the microwave to heat up the milk and then filter it through a filter pump. This way did not work at all and caused most of the problems that I ran into. From the microwave over cooking the milk to the filter getting clogged do to the fat cells inside the milk. In the end Cori found a way to readjust the settings using the liquid settings on the autoclave machine and I ended up autoclaving the antibiotic-free skim milk.
Yes after waiting and doing some more waiting I actually got to physically start my project this week in lab. All my supplies finally came in to make the L. S. Differential media I’ll be using in my project. On Monday I talked to Cori about how long it would take from start to finish. She told me that it would take about three and half hours. It was a no go on Monday for making the media because I wouldn’t have enough time to finish.
On Wednesday I came into the lab and made the media. I’m not going to lie it took me more than three and half hours to make the media. However, it was a great learning experience. I ran into a few problems making the media but nothing I couldn’t overcome with the help of Cori. The first problem that came up had to do with adjusting the timer on the autoclave machine. The L. S. Differential media requires antibiotic-free skim milk to be added into the main media along with TTC (Triphenyl-Tetrazolium Chloride). In preparation the antibiotic-free skim milk had to be autoclave for five minutes and the setting on the autoclave are set at fifteen minutes. When we asked Josh about how to go about changing the settings he brought up another way to prepare the skim milk instead of changing the settings. That way was to use the microwave to heat up the milk and then filter it through a filter pump. This way did not work at all and caused most of the problems that I ran into. From the microwave over cooking the milk to the filter getting clogged do to the fat cells inside the milk. In the end Cori found a way to readjust the settings using the liquid settings on the autoclave machine and I ended up autoclaving the antibiotic-free skim milk.
Thursday, October 2, 2014
The Waiting Game
After doing some research last Friday I came upon two different test solutions that would simulate gastric fluid and intestinal fluid. I took my research to Matt to make sure it was doable inside the lab and it turns out it is. Matt had to order a few thing to make it possible to make the fluids inside the lab which should be coming in sometime next week. So as of right now I am just waiting around for the items to come in but I have been using this time to type out my abstract, background information and procedures. It’s never too early to start thinking about the rough draft paper that will be due next month as we get closer to the end of the semester.
Image is from http://www.firstcovers.com
Thursday, September 25, 2014
The digestion process - What happens to your food as it travels through ...
This week in lab I worked on getting the knots out of my project that keep me from getting it off the ground. After brainstorming for what seem like forever I came up with a plan that I’m happy with and most important I got the okay on. I’m going to be testing the effect of stress that probiotics go through inside the digestive system to see the survival rate. The two probiotics that I’ve chosen for this project will be Lactobacillus bulgaricus and Streptococcus thermopilus these two probiotics are mostly found in yogurt… Yep that’s right I’ll be using yogurt once again however this time around I’ll be only using three yogurt products instead of twenty products.
So the first thing I’ll be doing is testing each product to make sure they contain the probiotics I’m looking for. For test one I want to use LS differential agar but we don’t have in the lab. I guess I’ll have to talk with Matt to see if we can order it. This media would be able to differentiate between Lactobacillus bulgaricus and Streptococcus thermopilus. In the mean time I’ve been talking with Cory to see what else I could use that we already have inside the lab. After I get done with test one I’ll be moving on to the stress part of the project. Taking the probiotic and putting it into an environment that is like the human stomach. It looks like I’ll be use hydrochloric acid because it’s very close to the pH level of the stomach. I’m still thinking of what other material I’ll be using to create a similar environment of the stomach.
Video from McGraw Hill on youtube.
Thursday, September 18, 2014
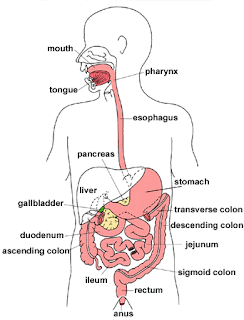
Breaking out the anatomy textbook
This week I have been researching how the digestive system works and mapping out the probiotic project. After talk to both Matt and Josh I got an understanding of where I want to go with this project and how I can accomplish it. The base of my project will be how different products with probiotics hold up in the digestive system. I will be measuring to see which product will have the most lactobacillus produced.
My first step was doing research on how food get digested in the stomach and moves on to the small intestine. While researching I also found out the pH levels of the stomach and small intestine. By getting this information I am trying to come up with a media that will be similar to the gastric acid in the stomach. There are other items that still need to be mapped out but I am happy to say that the probiotic project is coming together.
When it comes to the products I’ll be using I have narrowed it down to five products. I was going to use a pill supplement but after talking to Josh about it I decided to can that idea. There are just too many supplements out on the market. Instead I am going to stay with buttermilk and yogurts (three different types - homemade, Greek yogurt, plain yogurt). I will also be adding kefir into the mix. Kefir is a fermented milk drink made with kefir grains and has its origins in the north Caucasus Mountains. It is prepared by inoculating cow, goat, or sheep milk with kefir grains.
Image from http://www.aspirus.org
Subscribe to:
Posts (Atom)